Crystallisation in Confinement
The goal of this theme was to develop an understanding of how confinement affects crystallisation, which will ultimately enable us to exploit confinement in order to control crystallisation. Many crystallisation processes of great environmental, technological and biological importance, such as the formation of biominerals, weathering and frost heave, and the templating of nanostructures occur in small volumes rather than in bulk solution. There is much experimental evidence that shows that confinement can be used to define the orientation, polycrystallinity and morphology of crystals. Importantly, it can also have remarkable effects on crystal polymorph, stabilising metastable phases such that polymorph selection can be achieved by changing the confinement volume. However, understanding of many of these effects is poor.
Review Article: “Crystallization in Confinement” in Advanced Materials
Meldrum F.C. and O’Shaughnessy C. “Crystallization in Confinement” Advanced Materials, (2020), 32(31), 2001068.
Abstract: Many crystallization processes of great importance including frost heave, biomineralization, the synthesis of nanomaterials and scale formation occur in small volumes rather than bulk solution. This review article describes the influence of confinement on crystallization processes, where it draws together information from fields as diverse as bio-inspired mineralization, templating, pharmaceuticals, colloidal crystallization and geochemistry. Experiments are principally conducted within confining systems that offer well-defined environments, where these can vary from droplets in microfluidic devices, to cylindrical pores in filtration membranes, to nanoporous glasses and carbon nanotubes. Dramatic effects are observed, including a stabilization of metastable polymorphs, a depression of freezing points, and the formation of crystals with preferred orientations, modified morphologies and even structures not seen in bulk. Confinement is also shown to influence crystallization processes over length scales ranging from the atomic to hundreds of micrometers, and originate from a wide range of mechanisms. The development of an enhanced understanding of the influence of confinement on crystal nucleation and growth will not only provide superior insight into crystallization processes in many real-world environments, but will also enable this phenomenon to be used to control crystallization in applications including nanomaterial synthesis, heavy metal remediation and the prevention of weathering.

Calcium Sulfate Precipitation in Track-Etched Membranes
Galloway J.M., Aslam Z.P., Yeandel S.R., Kulak A.N., Ilett M.A., Kim Y-Y, Bejarano-Villafuerte A., Pokroy B., Drummond-Brydson R.M., Freeman C.L., Harding J.H., Kapur N., Meldrum F.C. “Electron transparent nanotubes reveal crystallization pathways in confinement”, Chem. Sci., (2023), 14(24), 6705-6715.
The cylindrical pores of track-etched (TE) membranes offer excellent environments for studying the effects of confinement on crystallization. These are available in a wide range of pore diameters and have simple shapes. While single crystals of a range of compounds including calcite, vaterite and aragonite and hydroxyapatite form in the pores of TE membranes, the inability to study the crystals in situ within the pores means that the mechanism by which the single crystals develop is unknown. As such, we developed a strategy to study the evolution of crystals in confinement by coating the porous membranes with a thin layer of amorphous titania prior to mineralization. Subsequent dissolution of the membrane then releases electron-transparent nanotubes containing calcium sulfate rods, where the titania provides mechanical stability for early stage and fragile precursor particles.
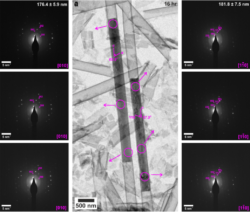
Figure 1: TEM images and corresponding SAED patterns of calcium sulfate precipitated within 200 nm diameter (manufacturer quoted) TiO2 nanotubes after (a) 16 hr. Area selected for diffraction is indicated by circles on TEM images, arrow indicates corresponding SAED pattern. Gypsum reflections are labelled in pink, and consistently have the c axis aligned parallel to the long axis of the crystals.
These studies have revealed that gypsum crystals formed in the 200 nm pores and were either single crystal or oligocrystalline, where the long axis of the rod corresponded to the rapidly growing [001] axis of gypsum. The crystals formed in the 100, 50 and 25 nm pores, in contrast, were single crystals of bassanite and exhibited long axes corresponding to the [110] direction. Anhydrite formed in the 10 nm pores. By comparison, gypsum formed in control experiments conducted in bulk solution. By visualizing the evolutionary pathways of the crystals within the pores we have shown that bassanite formed before gypsum in the larger 200 nm pores and that multiple nucleation events occur within each nanotube. This suggests that an Ostwald ripening process may occur within the pores to give single crystal products as the crystallization fills the confines of the pores. The orientation of the bassanite and gypsum crystals was also established at early times, which indicates that the pore surface is likely to be responsible for orienting the crystals in confinement.
Finally, the transformation of bassanite to gypsum within the membrane pores was studied using experiment and potential mean force calculations and was shown to proceed by localized dissolution/ reprecipitation. This work provides insight into the effects of confinement on crystallization processes, which is relevant to mineral formation in many real-world environments.
In-situ Study of Calcium Sulfate Precipitation in Controlled Pore Glass
Anduix-Canto C., Levenstein M.A., Kim Y-Y, Godinho J.R.A., Kulak A.N., Gonzalez C., Withers P.J., Wright J.P., Kapur N., Christenson H.K. and Meldrum F.C. “Exploiting Confinement to Study the Crystallization Pathway of Calcium Sulfate” Adv. Func. Mater. (2021) 31(50), 2107312.
Here, we have explored an in situ, imaging-based strategy that exploits confinement effects to study crystallization pathways. Crystallization is conducted within millimetre diameter nanoporous controlled pore glass (CPG) rods such that the developing crystals are fixed in position. In situ synchrotron X-ray micro-computed tomography (μ-CT) and X-ray diffraction computed tomography (XRDCT) were then used to follow their spatial and structural development in 3D, where the extreme confinement offered by these environments significantly retards crystallization, such that intermediate phases that are short-lived and potentially overlooked in bulk solution are readily observed. This approach was applied to the precipitation of calcium sulfate, where this was achieved by inserting a wet CPG rod between two sealed tubes, one containing 3M CaCl2 and one containing 3M (NH4)2SO4 solutions. Counter-diffusion of the calcium and sulfate ions leads to a time-dependent development of supersaturation within the rod.
A diffuse band corresponding to an amorphous form of calcium sulfate initially formed, and subsequently transformed to bassanite (CaSO4.0.5H2O). No transformation to gypsum occurred over the entire 3 week duration of the experiment. The stabilization of bassanite in purely aqueous environments at room temperature for such long periods is unprecedented. By comparison, gypsum formed within seconds in control experiments conducted with a glass tube in place of the CPG rods.
The development of particles within the CPG rods was observed in situ using μ-CT and XRDCT, where this reveals how the population of individual particles develops in 3D over time. Analysis after 3h revealed spheroidal bassanite crystals that were tens, to several hundreds of microns in size, and the number and sizes of the crystals continued to increase with time. The influence of the CPG surface chemistry in directing crystal growth was also investigated by precipitating calcium sulfate within CPG rods whose surfaces had been functionalized with carboxyl-terminated monolayers. Little difference was seen in the rate of crystallization as compared with unfunctionalized rods, but the amorphous phase now transformed to both bassanite and gypsum. Notably, the gypsum crystals either form de novo in the glass matrix or very close to existing bassanite crystals. In no case did the growing gypsum crystals induce dissolution or transformation of bassanite crystals in their vicinity.
These measurements therefore conclusively demonstrate that calcium sulfate can precipitate as an amorphous phase and bassanite at room temperature, and that concurrent pathways can operate in aqueous solution, such that the amorphous precursor phase can transform into either bassanite or gypsum according to the local environment. This work also fore offers a novel strategy for studying crystallization pathways and demonstrates the significant impact of confinement on calcium sulfate precipitation, which is relevant to its formation in many real-world environments.

Figure 2: Calcium sulfate precipitation in a carboxylate-functionalized CPG rod. (A) A 2D µ-CT slice of crystals precipitated after 2h 30min, where the brighter circular crystals are bassanite and the darker, dendritic crystals are gypsum. (B and C) Diffraction tomography, where (B) is plotted using the (110) reflection from bassanite and (C) the (020) reflection from gypsum. Different samples are shown for (A) and (B-C), where the amount and distribution of the mineral deposits varies from slice to slice.
