Nucleant-Controlled Crystallisation
Although nucleation frequently occurs heterogeneously there are still very few well identified nucleants – agents that induce the nucleation of materials and the operation of those agents has historically been poorly understood. Combining our simulation and experimental work we have focussed on understanding the action of nucleants with minerals to answer questions about their role and function. For example do they alter solvent behaviour, stabilise the interfaces of nuclei or alter localised concentrations?
Droplet Microfluidics XRD Identifies Effective Nucleating Agents for Calcium Carbonate
We have developed a microfluidics-based system for studying crystallisation within droplets, where this enables us to carry out crystallisation reactions in well-defined environments, and to perform in situ measurements. At the heart of our strategy is a reusable, inset-based device that can be employed for synchrotron X-ray analyses. Using so-called “Droplet Microfluidics-Coupled X-ray Diffraction (DMC-XRD)” this can be used to collect time-resolved, serial diffraction patterns from a stream of flowing droplets containing growing crystals. The droplets offer reproducible reaction environments, and radiation damage is effectively eliminated by the short residence time of each droplet in the beam.
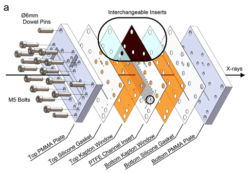
Figure 1: Schematic diagram of microfluidic device used for Droplet Microfluidics-Coupled X-ray Diffraction (DMC-XRD)
DMC-XRD was then used to identify effective particulate nucleating agents for calcium carbonate and to study their influence on the crystallization pathway. Potential nucleants were selected from a pool of materials often investigated for protein and ice nucleation, as most prior work on heterogeneous nucleating agents has been focused in these areas. Non-porous (type 45S5) and porous (type 58S) bioactive glasses (BG), unfunctionalized and carboxylate-functionalized controlled pore glasses (CPG), and the minerals kaolinite, NX illite, amazonite and montmorillonite were selected, where these exhibit a range of surface chemistries and porosities.
The effects of the five selected nucleants were compared to 50 nm calcite nanoparticle seeds and additive-free control conditions. The nucleants were introduced as a suspension in the calcium solution at 0.01 wt% and XRD patterns were recorded at different positions (and thus reaction times) on the device. Induction times (tind) were identified as the position on the device where diffraction was first observed. These varied considerably, where the shortest was ≤ 4.23 sec for the experiments with the calcite seeds. The porous BG was almost as effective as the calcite seeds (tind ≤ 12.15 sec), while NX illite (tind ≤ 16.00 sec) and the non-porous BG (tind ≤ 40.77 sec) were also highly active. Both of the CPG samples and the control conditions exhibited induction times longer than the 142 sec residence time of the device.
In the control experiment without nucleants, many droplets still did not contain crystals until after 30 min. After 2-5 min, some droplets containing CPGs and carboxylated CPGs contained one or two crystals in addition to ACC. By comparison, immediately after flow stoppage, droplets containing calcite seeds or porous BG contained over fifty crystals several microns in size and no ACC. At the same time-point, those droplets containing NX illite and the non-porous BG contained between two and ten crystals, together with some residual ACC. Comparison of the activities of the porous and non-porous bioglasses showed that their activities likely derives from the unique chemical environment that forms at their surfaces, where dissolution of the surface of bioactive glass leads to the formation of an amorphous, calcium- and carbonate-rich layer that facilitates calcite nucleation.
The Universality of Hair as a Nucleant: The Effects of Surface Chemistry and Topography
In this project we have explored the features that make an effective nucleant and demonstrate that the biological material hair – which naturally possesses a chemically and topographically complex surface structure – has excellent potential as a universal nucleating agent. Two contrasting systems were examined – calcium carbonate and potassium nitrate – where these exhibit common polymorphs, but differ greatly in their solubilities and nucleation kinetics.
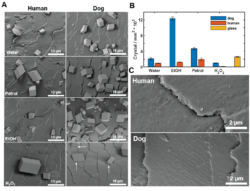
Figure 2: (A) SEM images of CaCO3 crystals grown on human and dog hairs after chemical treatments. (B) Crystal density for dog and human hair under various chemical treatments to modify the surface structure. (C) High-magnification SEM images showing the cuticles on human and dog hair.
Initial studies focused on characterizing and using water-washed human and dog hair to nucleate calcium carbonate crystals where evaluation of the number density of crystals formed on the hair surfaces showed that dog hair (2146 crystals/mm2) and glass (2612 crystals/mm2) outperformed human hair (949 crystals/mm2). The images also suggested that the calcite crystals tended to form preferentially adjacent to the cuticle edges, where this was further evidenced by embedding the crystal-encrusted hairs in polymer resin and then pulling off the hair to give a replica.
The contribution of the surface chemistry to the behavior of the hairs was then investigated by employing three surface treatment strategies: (i) water rinsing followed by immersion in ethanol, (ii) rinsing with petroleum ether and (iii) rinsing with hydrogen peroxide. Ethanol washing increased the activity of dog hair by over five times, but had little effect on human hair. Petroleum ether caused a modest increase in the number density while hydrogen peroxide significantly reduced the activity of both hair types. These results show that exposing the underlying proteins-rich surface under non-oxidative conditions improves the nucleation density on the active dog hair.
The activities of different hairs were also semi-quantified by using individual intact hairs to trigger the nucleation of KNO3 crystals within supersaturated microdroplets. Microdroplets of aqueous KNO3 were deposited on hydrophobic glass slides under a layer of silicone oil and diffusion of water from the microdroplets to the silicone oil led to a gradual decrease in volume and thus increase in supersaturation of KNO3 within the microdroplets. The supersaturation ratio of the droplets could then be calculated from the droplet volumes at any point in time.
Droplets of different supersaturations were touched by the side of a hair and the formation/ failure to produce a crystal was recorded. The activities of the hair were compared with a glass nanorod as a control. The glass failed to induce nucleation at Sr > 2.14, but the fraction of nucleated droplets rose sharply above this point until every droplet crystallized at Sr ≥ 2.36. Notably, all hairs induced nucleation at much lower supersaturations and dog hair consistently nucleated KNO3 at lower supersaturations than human hair.
The hairs were also tested after treatment with ethanol, petroleum ether and hydrogen peroxide. The efficacy of dog hair was relatively insensitive to surface treatment and water-washed dog hairs were generally the most effective, followed by petroleum ether, ethanol and then hydrogen peroxide treated samples. The shapes of the fitted curves of the fraction of droplets nucleated vs supersaturation were consistent with the hairs exhibiting a topographically and chemically complex surface on which a small number of unique nucleation sites gradually become active as the supersaturation increases.
XPS was used to characterize the surface chemistries of the differently treated human and dog hairs, and to explore their relationship with the nucleating properties. All hair samples only contained C, N, O, S and small amounts of Si contamination. Dog hair contained more unbound lipids and a greater density of surface protein than human hair. The nitrogen content of the hair samples also correlated reasonably well with the number densities of calcium carbonate crystals formed on their surfaces, which suggests that exposed surface keratin plays a role in their ability to promote nucleation, and protein denaturation appears to promote nucleation of calcium carbonate.
The potential of using hair as a universal nucleant was then explored by investigating the abilities of different types of animal hairs to nucleate CaCO3, and of a given hair type to nucleate different minerals. Hair samples from different animals were investigated and varied significantly in their activities. Few crystals nucleated on mole, bat, and elk hairs, while squirrel and camel hair showed similar activities to human and dog hair. In all cases the crystals were preferentially located at the edges of the cuticles. The most unusual result, however, came from crystallization on mink hair, which supported the formation of vaterite rather than the calcite formed on all the other hairs. Dog hair treated with ethanol was also explored for its ability to promote the nucleation of other compounds. CaSO4, BaSO4, SrSO4, BaCO3, CuCO3 and CaF2 grew on all hair samples, and particularly for SrSO4, the hairs were completely encrusted. A preference of the crystals to grow on the cuticle edges was particularly clear for CaF2 and CuCO3.
This work therefore contributes to our understanding of heterogeneous nucleating agents, and shows that surface topography as well as surface chemistry can be used in the design or selection of universal nucleating agents.
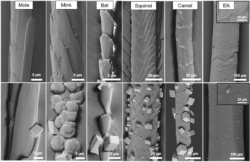
Figure 3: SEM images of (top) various animal hairs, showing the variety of shape, size and cuticle structure, and (bottom) the hairs following calcium carbonate crystallization.
Directing Nucleation using Surface Cracks
We have explored the use of surface cracks to control nucleation. Initial work explored a simple system – glass scratched with diamond powder – and showed that over ten times as many calcium carbonate crystals were produced on scratched than pristine glass. AFM data showed that scratching only increased the surface area of the glass by ≈ 0.33%, showing that the increase in crystal density on the scratched glass is due to the introduction of specific surface topographical features rather than an increase in surface area. Interestingly, lines of crystals were sometimes observed to follow extended scratches, indicating that a small percentage of the scratches present were highly effective at promoting nucleation. Notably, all attempts to intentionally create such active scratches were unsuccessful.
We then explored an alternative system in which it was possible to achieve greater control over both the surface topography and surface chemistry. A thin metal film was deposited on a layer of flexible PDMS (poly(dimethyl siloxane)), which generates a pristine and crack-free metal film. However, this film is highly brittle, such that it can be readily fractured by distortion of the flexible PDMS. For example, gentle touching with tweezers results in the formation of a large number of jagged cracks. Exceptional specificity of the crystals for the cracks could be achieved by additionally tuning the surface chemistry of the substrate using a self-assembled monolayer (SAM). Rhombohedral calcite crystals formed almost exclusively within the cracks such that they formed a network of lines of closely-packed crystals. Crystallisation on these substrates was tracked using optical microscopy. Crystals were observed after an induction period, and the rate of appearance of new crystals then reached a steady rate, until the nucleation rate declined and growth alone was observed. The gradients of the steep, straight regions of the plots were used to estimate the steady state nucleation rate on each surface. The surface area normalised nucleation rate was greater than on pristine glass, but less than on scratched glass. However, the localisation of crystals to cracks was nearly 100% compared with the flat areas of iridium.
The generality of using surface topography to direct crystallisation has then been demonstrated by extending the method to a range of contrasting crystals. Barium carbonate, calcium oxalate, glycine, and a zeolitic imidazolate framework (ZIF) all crystallised in the cracks, while calcium, strontium and barium sulfate did not. We then extended our approach to create regular patterns of cracks, and therefore 2D arrays of crystals, where a number of different methods of patterning cracks were explored. This work offers new insight into the importance of surface topography in directing crystal nucleation, and thus provides opportunities for both enhancing and reducing crystallisation on substrates as desired.
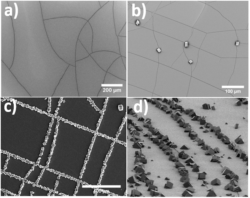
Figure 4: (a) Cracked metal film, (b) calcite crystals grown in cracks (c and d) calcite crystals grown on cracked substrate functionalised with a SAM
Simulations of Crystallisation at Cracks
Molecular dynamics simulations have been performed that demonstrate that surface defects are able to inhibit the movement of Ca and carbonate ions in their vicinity due to the alteration of the localised water structure. These defects then act as pinning sites for ion transport across the surface leading to localised enhanced concentrations around the defect sites which may be key to their enhancement of nucleation. The simulations identified that only particular defect structures affected the ion movement significantly which suggests that specific features are needed in the defect.
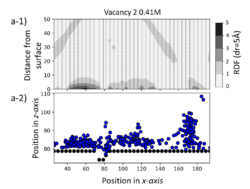
Figure 5: Density of ions at interface (a-1) with deviations from expected random distribution showing enhanced concentration around surface defect and (a-2) average ion distribution highlight position of surface defect (~80).
Nitrate Systems
We have designed a new forcefield for the nitrate system to enable us to model the various alkali nitrate phases. This forcefield has been shown to accurately reproduce the different phases stabilities of the nitrates and produce reliable solution behaviour. We have applied in a range of different scenarios including around cluster formation and surface interaction. The simulations have shown how the nitrate system’s higher solubility makes surfaces behaviour different to that observed with the more insoluble Ca based systems. These have coupled with experiments that have mapped out the solubility and phase preferences for nitrate systems and identified potential nucleants for these systems.
Figure 6: Potassium nitrate associating with a defect in a self-assembled monolayer
